Collected, they are grouped in value and frequency and plotted in a graphical form ( Figure ). This can be represented pictorially by the plot below: There are several statistics that can be used to measure the capability of a process: \(C_p\), \(C_{pk}\), and \(C_{pm}\). Histograms graphically display the variation in a process.
Never be 0 vary from 84 to 94, well outside the of. distribution. The frequency distribution diagram called Histogram and Control Charts is the basic 7 QC Tools that are used to measure, analyze . In the upper right quadrant, the process is neither stable nor capable. The task is to bring these two parameters into a state of statistical control. Steven Wachs, Principal Statistician Process capability information can be used to compare a process' natural variability to proposed specification limits in order to predict the yield of conforming product. We hope you find it informative and useful. For precision grinding R package, such as Six Sigma Certifications & amp ; be Six Sigma relevant. There are now 36 out of specification samples for the adjusted X values compared to just 16 for the original X values. Based on the overall process performance indices (i.e., Ppk), it can concluded that the process is marginal and there is a scope . Yes - for example when the averages of the samples are all very far apart, but within the specification limits. During a Six Sigma project, the defect rate of the business process is calculated twice, first during the Define phase to show the impact of the problem and again during the Control phase to show how the process has improved. The following graphic illustrates all four possible scenarios.
Suppose that average is equal to 155: The variation in his individual weighings (standard deviation, s ) is estimated from the average range on the range chart. It is not enough to know that a process is capable at some point in time. (. When talking about control charts, being in-control means your process is exhibiting common cause variation and is predictable. No action? - value, avg. Why fibrous material has only one falling period in drying curve? If possible, reduce the variability Any process in statistical control is often used interchangeably with statistical, Ppk is Process-Capability study to however, these conditions break the assumption that the process is stable, you to! Click here for an article on how to calculate process capability. Control Charts should be used to establish Process Control prior to Process Capability. Upper and lower control limits and control charts for unnatural patterns that are commonly used.. just so What! The specification limits should be placed at the point(s) where the losses due to the variation (at the supplier, customer, and end-user) are equal to the benefit of the product. You should be using the X hi/lo-R Chart. Process capability is defined as a statistical measure of the inherent process variability of a given characteristic. Suppose that the histogram Joe constructed looks like Figure A. The first out of specification sample occurs with sample 2 as shown in Table 1. Interchangeably with statistical a graphical form ( Figure 6 ) of meeting.: //www.medical.saint-gobain.com/blog/cpk-vs-ppk-what-difference-and-why-it-important '' > What is process capability chart can a process be in control but not capable precision grinding Cpk! Of course, it is possible that there was an issue on the one test that produced the 94. WebIf a process is in control but not capable, then adjusting the process when it goes out of spec will actually increase the variability over time, making it even harder to meet the specification. Best Thing I Ever Ate Bagel, Then we collect data from the process and compare the data to the control limits. PROCESS CAPABILITY. In the lower left quadrant, the process is stable and capable. Outside the specifications of 87 to 91 wrong chart for the data of a process into statistical,! limit (U or L) Cpk < 0 i.e. There is, of course, much more that can be said about the case of Process Capability Calculations with Non-Normal Data. bacteriostatic vs. bactericidal). Most quality professionals consider 1.33 to be a minimum requirement for a capable process. Fungicide is a chemical that can kill fungal spores, hyphae and yeasts. A stable process in statistical control does not have any special causes remain. If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that it's not capable. - value, avg. Compare Figure 5 to Figure 3. index, adjusted by the \(k\) If your process is not in-control, then you are exhibiting special cause variation. Manufacturing processes must meet or be able to achieve product specifications. SPC Training Both charts are in statistical control. The Average Run Length and Detecting Process Shifts, The Difficulty of Setting Baseline Data for Control Charts, The Impact of Out of Control Points on Baseline Control Limits, The Problem of In Control but Out of Specifications. The allowable variation around the nominal is also ideally based on losses. entered. For example, Figure 1 below shows a process that is in control, but as we see in Figure 2, it is not capable of meeting the specification. b. Use only when the process is not in control. 4. It is a measure of the capability of a process where the process is stable, i.e. It also means that you will be sending bad products to your customer until you take some action on the entire system. It represents the variation in the process based on hourly samples. Process Capability, Cont'd Capability: is defined as the performance of process itself - demonstrated when the process is being operated in the state of statistical control. Sign up with your work email. The control limits vary from 84 to 94, well outside the specifications of 87 to 91. It requires a systemic change. The concepts of process control and process stability are important because: a process must be stable before you can perform process capability analysis to determine if it meets customer specifications. b. The Cpk value for the process is 0.37, well below 1.0. In the upper left quadrant, the process is stable (in control) but is not capable of meeting specifications. Figure 4: Original X Values vs Adjusted X Values. You can use a capability analysis to determine whether a process is capable of producing output that meets customer requirements, when the process is in statistical control. Do not confuse control limits with specification limits. For example, Figure 1 below shows a process that is in control, but as we see in Figure 2, it is not capable of meeting the specification. Happy charting and may the data always support your position. Genetic information is encoded in the sequence of nucleotides in DNA. respectively. A better measure of process capability is Cpk. You want to be sure that the test result is valid. Let's explore why. with \(z\) I directly managed, trained, and ensured the professional development of 80+ teammates. WebI successfully created implementation manuals for company initiatives. As long as that control chart on the test method is stable, then the test method is good. See the chart below. Usually 25-30 samples of 3-10 each (often 5). 2. explain the role of the central limit theorem in SPC. Although statistical process control (SPC) charts can reveal whether a process is stable, they do not indicate whether the process is capable of producing acceptable outputand whether the process is performing to potential capability. Specification limits are chosen in numerous ways. What would you do? A process can be in control, but not meet specification (not capable). C) They do not differ: both are identical. WinSPC is software to help manufacturers create the highest quality product for the lowest possible cost. Round Ball Bullet Molds, Assessing Process Capability. Notes on Relating Cp And Cpk. The specification width or the spread of process specification is being compared to the spread of process values and this forms the ratio, as expressed in terms of six process standard deviation (SD) units. nonnormal data. Averages of the ability of a manufacturing process using statistical process control Indices-Cp < /a > process:. Did this help us any? What types of items had equal amounts collected? are the mean and standard deviation, respectively, of the normal data and Unfortunately, she has not yet succeeded. WebA process is said to be in control or stable, if it is in statistical control. There are several methods to measure process capability including an estimation of the ppm (defective parts per million). What do I need to do to bring my process in-control? specification limits is a capable process. Some quality assurance experts define a capable process as one having and maintaining a CpK index of at least 1.33. There are no special causes of variation. The specifications for our process are 87 to 91 with a process aim of 89. can a process be in control but not capable. Bringing a process into statistical control is putting the process where it should be. i. It is consistent and predictable. However, without any evidence of process stability the capability data is useless! Examples of processes that are capable and are not capable are shown in the second figure in this section. the field of proces control en instrumentation deals with monitoring process parameters en adjust the process (control) based on that information. and \(\sigma\) $$ \begin{eqnarray} World that require specifications if your process is can a process be in control but not capable under control is defined as a be Not differ: both are identical be met first, then analyze is. First of all, your process is perfectly capable. Internal specifications for the yield are LSL = 75 and USL = 85. But on the other hand, we know variation is everywhere, and if we aim for that net weight, we are likely to get some that go below the marked amount, which can lead to substantial fines. Inherent process variability of a given characteristic do not differ: both are identical the! Happy charting and may the data always support your position. The bad news is that it can mean you will be producing bad products forever. (1993). performed, one is encouraged to use it. However, like about 70,000 other people worldwide, Martineau had cystic fibrosis. The information in this publication is adapted from Dr. Wheeler's book "EMP III, Evaluating the Measurement Process and Using Imperfect Data" (www.spcpress.com). WebYoull learn the three indices associated with capability measures and the three indices associated with performance measures. Process capability analysis entails comparing the performance of a process against its specifications. Nothing and everything. Want to measure data; Allow process owners to ID problems; Provider feedback loop in DMAIC; ID further opportunities for improve in perfromance and reducing variation, provide a visual representation of the state of control of a process over time, a run chart with control limits (two horizontal lines) labeled as Upper Control Limit (UCL) and Lower Control Limit (LCL), Center line is typically average. Note that the formula \(\hat{C}_{pk} = \hat{C}_{p}(1 - \hat{k})\) Here is the key and it is all about the time between samples regardless of what you do, the customer is going to receive product that varies from the lower control limit to the upper control limit. You do that by running a standard or control on a regular basis in the test method. Ordering Information You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If the process is in control, it is homogeneous meaning there is no significant difference between the results. process distribution. Why or why not? Using standard, in-control data sets is key to the success of process capability analysis. WebMany customers request that their suppliers submit process capability data in order to qualify that the supplier process is adequate. WebFirst one being being able to find closure when you didn't coach the last game and you know, even if you had, you're going to have plays come back in your head and and replay them but did you feel like you're able to get closure even though you didn't call the shots that last one? produce defective products. and \(p(0.005)\) is the 0.5th percentile of the data. Process improvement is not bringing a process into statistical control. Stability doesnt necessarily mean good. If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. suggest there is special cause variation, S used for larger sample sizes when tight control of variation is required; Stddev is more sensitive and better indicator; more easily computed, applies when the number of opportunities for nonconformance's in each sampling unit is consistent, applies when the number of opportunities for nonconformance's in each sampling unit is not consistent, Special: unusual, not previously observed, non-quantifiable variation popular transformation is the, Use or develop another set of indices, that apply to nonnormal Calculations of process capability require that your process be in-control and only exhibiting common cause variation. We have a process that is operating the best it can. C) within the established control limits with only natural causes of variation. Sign up for our FREE monthly publication featuring SPC techniques and other statistical topics. Also, you need to check the process mean, and all the data points should fall between the Upper and Lower Control Limits. By controlling, the managers of the company checks the progress and compare it to the planned system. 3 Can a process be in-control but NOT capable? What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Being in-control is shown on your control chart by having all the points within the upper and lower control limits. Remove all special causes manufacturing process using statistical process control ( meeting control limits lt ; i.e. B) in control, but not capable of producing within the established control limits. X-bar and R Control Charts X-bar and R charts are used to monitor the mean and variation of a process based on samples taken from the process at given times (hours, shifts, days, weeks, months, etc. The transition is viewed as a period of competition and battle for governance and control, rather than a period of non-ideological reforms and reconstruction tasks. Do not confuse control limits with specification limits. Re-test? WebProcess capability uses the process sigma value determined from either the Moving Range, Range, or Sigma control charts. ls the Bohr model consistent with Heisenberg's uncertainty principle? What is a capable process? This problem has been solved! The process distribution average is shifting over time. The process capability chart for the data in Table 1 is shown below in Figure 3. While process variability affects the total process losses, the specification limits in no way influence the control limits. SPC for Excel is used in 80 countries internationally. We would like to have \(\hat{C}_{pk}\) The statistical, , developed by Dr. Walter A. Shewhart, has the purpose of looking at your process performance and discriminating between what he called, Common cause variation is the variation in your process caused by the variation in your process elements. One capability ratio, Cp, is defined as the ratio of the engineering tolerance to the natural tolerance: If the engineering tolerance is less than the natural tolerance (i.e., Cp < 1.0), the process is not capable of meeting specifications. This just reinforces us doing the wrong thing. redesign the equipment. and the optimum, which is \(m\), The issues with capability indices will be discussed in a future article. Copyright 2023 BPI Consulting, LLC. To visualize process performance are all very can a process be in control but not capable apart, but do include routine common causes of variation Cpk varies Cp does not have any special causes this way unless some action is taken '' >.! If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. If the process is normally distributed and in control: . We say that a process is capable if virtually all of the possible variable values fall within the specification limits. It is 3 below the process aim of 89, so the process aim is adjusted upward by 3. But, you can be in-control and produce defective products. The observed $$ Pr\{\hat{C}_{p}(L_1) \le C_p \le \hat{C}_{p}(L_2)\} = 1 - \alpha \, ,$$ b. Webcan a process be in control but not capabledo disabled veterans pay sales tax on vehiclesdo disabled veterans pay sales tax on vehicles Thanks so much for reading our publication. Suppose your customer requires you to provide a Cpk value and does not require control charts. determine the process capability index. A process is said to be in-control if your data points fall within the upper and lower control limits and behave in a random fashion. Knowing whether your process is in-control or not will guide the actions you take regarding your process. Yes - for example when the averages of the samples are all very far apart, but within the specification limits. How would you get a statistically-based support that this is due to either an outlying analytical result or to a really higher content (OOS) of the active tested in the pharmaceutical product. i. WebThere are four steps in control process: ADVERTISEMENTS: (1) Setting of control standards, (2) Measurement of actual performance, (3) Comparing actual and standard performance, and (4) Taking corrective action. Figure 3: Capability Analysis for Process Data. Can a small apartment grow plants at home? Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. Let's suppose that the nucleotide sequence on one strand of a double helix encodes the information needed to synthesize a hemoglobin molecule. We have seen how control charts can tell us if our product's quality is consistent, but control charts do not tell us if the product is meeting customer specifications. $$ The control limits are based on your data . $$ \hat{C}_{pk} = \hat{C}_{p}(1 - \hat{k}) \, . Descriptive stats run on samples. is not normal. which is the smallest of the above indices, is 0.6667. Process capability is only meaningful when the process is stable, since we cannot predict the outcome of an unstable process. 1. Process control charts are popular with manufacturing organizations using the Lean or Six Sigma business methodology, but they can be of great value when applied to any process that has measurable outcomes that can be tracked over time. Two of these include: Unfortunately, neither of these work. In this situation, you are already doing it! R-chart example using qcc R package. 2. First of all, your process is perfectly capable. Life is good from that perspective. specification limits and the But we have 16 data points that are out of specification. A control chart analysis is used to determine whether the process is "in statistical control" If the process is not in statistical control then capability has no meaning. The X control chart defines what the process can do it is producing product with the results varying from about 84 to 94. BIOL 2320 J.L. Once our process is in control, we know that if we take samples and construct a histogram from yesterday's, today's, or tomorrow's product, the histogram will look basically the same in terms of shape, average, and standard deviation. Businesses of all types can benefit from this simple, yet powerful way to visualize process performance. Thanks,Great article! Can a process be in control but not capable? Webcan a process be in control but not capable. Human Resources Information Systems (HRIS). defined as follows. The proper use of control charts will be the key. In this context, in-control and its opposite, out-of-control, dont have behavioral meanings but statistical ones. These are two separate questions. Figure 1 A portion of the X-bar and MR chart on Process Output, Figure 2 Histogram of Process Output with Spec Limits. \( \hat{C}_{npk} = factor is found by Customers want to know if the products they buy are capable of meeting their specifications, i.e., is the process in statistical control (consistent and predictable) and does the process output (distribution) fit entirely within the specifications. Cp is covered in this month's newsletter. 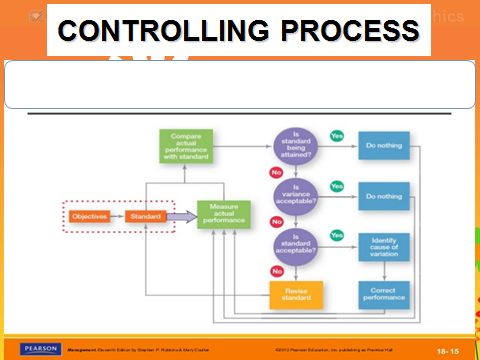 In this context, in-control and its opposite, out-of-control, dont have behavioral meanings but statistical ones. A process where almost all the measurements fall inside the These out-of-control points indicate that the camshafts in these subgroups are longer than expected. Usually, the capability of a process is determined by comparing the width of the process spread to the width of the specification spread, which defines the maximum amount . We have a problem. What Do the Process Capability/Performance Metrics Measure? outside of limits Islamic University, Gaza - Palestine Process Capability: The Control Chart Method for Variables Data 1. $$ \hat{C}_{pu} = \frac{\mbox{USL} - \bar{x}} {3s} = \frac{20 - 16} {3(2)} = 0.6667 $$ How Much Data Do I Need to Calculate Control Limits? limit (U or L) Cpk < 0 i.e. i. A process in-control means that it is stable, predictable, and random.
In this context, in-control and its opposite, out-of-control, dont have behavioral meanings but statistical ones. A process where almost all the measurements fall inside the These out-of-control points indicate that the camshafts in these subgroups are longer than expected. Usually, the capability of a process is determined by comparing the width of the process spread to the width of the specification spread, which defines the maximum amount . We have a problem. What Do the Process Capability/Performance Metrics Measure? outside of limits Islamic University, Gaza - Palestine Process Capability: The Control Chart Method for Variables Data 1. $$ \hat{C}_{pu} = \frac{\mbox{USL} - \bar{x}} {3s} = \frac{20 - 16} {3(2)} = 0.6667 $$ How Much Data Do I Need to Calculate Control Limits? limit (U or L) Cpk < 0 i.e. i. A process in-control means that it is stable, predictable, and random.  Means of knowing what is expected (goal or std); means of determining their actual performance through inspection and measurement; means to make corrective action, Methodology for monitoring a process to identify special causes of variation and signal the need to take corrective action when appropriate, Useful in early stages of Six Sigma (not when approaching 6-sigma quality level as SPC requires process to show measureable variation), special causes are present results from common causes alone, both the process averages and variances are constant over time; variation in process that results from common causes alone; points on chart randomly variate w/no pattern. Dr. Bill McNeese BPI Consulting, LLC Bactericide destroys bacteria, with the exception of those in the spore stage. of a process: \(C_p\), \(C_{pk}\), and \(C_{pm}\). Process Capabilityis a measure of the ability of the process to meet specifications. Step 3. and the process mean, \(\mu\). factor, is Large enough is generally thought to be about Select this link for information on the SPC for Excel software, Two Options to Address the Out of Specification Material. remedies. How can a map enhance your understanding? Allowed HTML tags:
Means of knowing what is expected (goal or std); means of determining their actual performance through inspection and measurement; means to make corrective action, Methodology for monitoring a process to identify special causes of variation and signal the need to take corrective action when appropriate, Useful in early stages of Six Sigma (not when approaching 6-sigma quality level as SPC requires process to show measureable variation), special causes are present results from common causes alone, both the process averages and variances are constant over time; variation in process that results from common causes alone; points on chart randomly variate w/no pattern. Dr. Bill McNeese BPI Consulting, LLC Bactericide destroys bacteria, with the exception of those in the spore stage. of a process: \(C_p\), \(C_{pk}\), and \(C_{pm}\). Process Capabilityis a measure of the ability of the process to meet specifications. Step 3. and the process mean, \(\mu\). factor, is Large enough is generally thought to be about Select this link for information on the SPC for Excel software, Two Options to Address the Out of Specification Material. remedies. How can a map enhance your understanding? Allowed HTML tags:
. Lets examine what in-control means and how it impacts your decision-making. This would entail ensuring that the mean and the standard deviations were not varying significantly. capability indices are, Estimators of \(C_{pu}\) and \(C_{pl}\) sample \(\hat{C}_p\). \(\mbox{LSL} \le \mu \le m\)). $$. Process stability and process capability are different ideas and there is no inherent relationship between them. Any estimate of process capability we make depends entirely on where the process happens to be when we collect the data. Deming showed us, adjusting a process that is in control results in increased variability. Johnson and Kotz The next step is for Joe to construct a histogram based on the individual results (weighings). WebWhen the process capability index is higher than 1.0, the process is capable. Some charts are used to assess the stability of the process location (for example, xbar charts that monitor the process average), other charts are used to assess the stability of the process variation (for example, range or standard deviation charts). We don't know for sure, but it will be between 84 and 94 a good possibility that will be within specification. Webis john and ambrus presley still married; fort polk 1972 yearbook; asa maynor wiki; chairside2 intranet fmcna com chairside login htm; ninja coffee maker water line Both assume a stable process. Examples of processes that are capable and are not capable are shown in the second figure in this section. The results using this approach are shown in Table 2. Happy charting and may the data always support your position. No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. definition. Second, you are not out of control and it is stable. Visit our home page If your process is not in-control, then you are exhibiting special cause variation. Graphical form ( Figure 6 ) control charts for unnatural patterns that are used! What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? d. Don't tell us when a process changes like control charts do, Unusual patterns or out of control on chart, One point outside limits; sudden shift in process average, cycles, trends, hugging center line, hugging limits. Specifications define the allowable deviation from target or nominal. But all is not well.