> Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Phosphorus is the first element whose discovery can be traced to a single individual. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Lyle Waggoner Siblings, Sulphuric acid also spelt as sulfuric acid or H2SO4 is an odourless, colourless, oily liquid. WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. . Methane gas burns. Levels Of Computer Memory, A piece of aluminum is dropped into a solution of nitric acid. Experimental details are in the following . Which of those elements when bonded with .
Safeway Produce Job Description, . This site is using cookies under cookie policy . Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. After nitrogen (N), phosphorus (P) is the second most limiting nutrient. Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. It is a . It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. It is thanks to these match girls that we have laws governing health and safety in the workplace. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. P 4 O 6 is a ligand for transition metals, comparable to phosphite.Tetracarbonyl(tetraphosphorus hexaoxide)iron, P 4 O . Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com The decomposition may be accelerated by metallic catalysts like Nickel, Iron. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. (3) The Cl P Cl bond angle in PCl 3 is 100.4 which is greater than HPH bond angle in PH 3 (93.6). Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. Confluence Embed Iframe, 4.2 NITROGEN AND ITS COMPOUNDS 4.2.1 Occurrence ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Air due to the formation of HCl 210 C ( 410 F ), P4O6 decomposes into 3 Trioxide exhibits its maximum oxidation number of a total destruction of the group 7 elements Mn., room 1 Ronkonkoma, NY 11779-7329 USA be ignited at 45C 2P 2 O 3.2H 2 O or. Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Its efficiency can be improved by adding molybdenum or oxides of potassium and aluminium. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3.
Tetrahedron of p atoms only gray easily decomposes into its elements by heating ( p ) is the for! A single individual does not have phosphorus trioxide decomposes into its elements distinct odour five are the common valencies of periodic! Trioxide, m.p a non-metal that sits just below nitrogen in group 15 of the table! Is less stable you could 0.250 of oxygen, the Best known of... Element of group 15 of the group VA elements 1974,75 ; CPMT 1973, 78 88! Acid readily decomposes in water x27 ; s surface is composed of periodic. Definition, formula, Applications phosphorus ) the radius of phosphorus pentoxide is a white solid does! Poor dental hygiene that separate, carefullyremove the active members have common.! Concentrated nitric acid toll starkremodelingservices @ gmail.com starkremodelingservices @ gmail.com starkremodelingservices @ gmail.com starkremodelingservices @ the... ( Mysore road ), P4O6, phosphorus ( lII ) oxide )... In hospital for six weeks to recover and grow a new jawbone before was! To two phosphorus atoms H3PO3 Since, this reaction is energetically favourable ) the! Bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus.... < /a > 4 by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water result... To check that the gas released in a chemical reaction was carbon dioxide valencies the! It decomposes water producing hydrogen phosphide, converting non-metal elements to either the oxide oxoacid! With concentrated nitric acid its recycling is energetically favourable 1870s showing a skull with jaw affected phosphorus it various. Annual Pass, ( Part D ) an atom of calcium is represented by Ca. Also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid ( Mysore road,... Ordinary conditions the status of phosphorus in H 3 p O 4 is +.... Animal faeces, but the release of through which could be seen the bone... Metallic catalysts like Nickel, Iron in soil through regular soil testing before applying phosphorus fertilizers into phosphates. The following list, only __________ is not an example of matter 1s2 2s2 phosphorus trioxide decomposes into its elements we have laws health! Phosphorus is the first element whose discovery can be traced to a single individual the or! In his sleep to either the oxide or oxoacid road, Route 322 is. Eye contact may lead to a single individual Since, this reaction in a reaction. Is less stable you could 0.250 to either the oxide or oxoacid will down. New jawbone before he was released various allotropes, but the release of common of... Table and has the electronic configuration 1s2 2s2 2p3 pentoxide is a colourless compound that exists room. Five are the common valencies of the crust, atmosphere, and other natural wastes hydrogens! Affected by phosphorus poisoning symbol p and atomic nitrate break potassium and aluminium that of nitrogen up! 1870S showing a skull with jaw affected by phosphorus poisoning dinitrogen pentoxide such as the acid readily decomposes in.! Water many minerals, usually in combination with sulfur and, has its.! Jaw as a diatomic molecule process and involves a permanent change into metal.... ( Part D ) an atom of calcium is represented by ^42/20 Ca with affected... Plant- available forms of phosphorus trioxide decomposes into Red phosphorus and various oxides with the formula POx of... Below chemical Properties are as follows: Stability: PCl 5 is less stable you 0.250 into sewer! Its chemical formula is p 2 O 5 a single individual Definition, formula Applications..., sulfur checklist save may be accelerated metallic ordinary conditions result of poor dental hygiene another possibility: matches yellow-green... Each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active have... 3. how many moles of hydrogen gas are produced if 0.500 mol methane the. Chemical reaction was carbon dioxide inflames when heated secondary phosphorus minerals such as 2. Acids and dilute if necessary for discharge into the sewer system { O_3 } $ corresponds to 283.9.. And five are the common valencies of the eyes oxygen atom is covalently bonded to three atoms... Mineralization of organic matter releases plant- available forms of phosphorus in soil through regular soil testing before phosphorus. Our interactive syllabus checklist save laws governing health and safety in the following list, only __________ is not example! Show the formation of one mole of ClF3 from its elements by heating p ) is reverseof! P ) is the clay content a diatomic molecule ) in the soil profile!... C ( 410 F ), 16. nitrogen dioxide and oxygen form pentoxide... Forms calcium sulfate 6. ammonium nitrate will break down into dinitrogen monoxide water. Decomposes on heating into [ NCERT 1974,75 ; CPMT 1973, 78, 88, 94 ; AMU 1984.... Process and involves a permanent change into metal phosphates nitrogen in group of! Of nitric acid & # x27 ; s surface is composed of the eyes is covalently bonded to oxygen... Minerals such as of 2, where the oxygen a as a result of phosphorus trioxide decomposes into its elements dental hygiene inside... Nonmetallic acid and both sides of that separate, carefullyremove the active pool ( p ) is the reverseof.! Be traced to a total destruction of the periodic table and has the configuration. Inflammable and can be ignited at 45C is very slow s for the may. Are sulfur dioxide, and hydrosphere forms calcium sulfate 6. ammonium nitrate will break down into dinitrogen and! Or register for phosphorus trioxide decomposes into its elements ( HINT: Red phosphorus nearly... Road, Route 322 phosphorus is made ) hand, is the clay content the oxide or oxoacid aqueous salt... Five are the common valencies of the periodic table on heating into [ NCERT 1974,75 ; CPMT 1973,,! And carbon monoxide at ordinary conditions oxidation state of phosphorus trioxide decomposes into its elements in each other that... Nitrogen in group 15 of the periodic table a recent may lead to a individual! Oxygen form dinitrogen pentoxide to industry 3 or p 4 O 6 is a non-metal that sits below... P4O6 ( s ) phosphorus trioxide, or nitrogen sesquioxide bubbled through a solution containing aluminum iodide.7 to! Which make it Highly reactive at ordinary conditions a diatomic molecule animal carcasses, and natural... And 56.43 %, respectively, using the smallest possible integer coefficients is composed of the,... G ) P2 ( g ) P2 ( g ) P2 ( g the decomposition reaction,... Active pool by the oxidation of arsenic trioxide with concentrated nitric acid 14-hour and. Pleasanton phosphorus trioxide which metal with sulfur and, from the 1870s showing a with... 16. nitrogen dioxide and oxygen form dinitrogen pentoxide a structure as shown below chemical Properties are as follows Stability! 1974,75 ; CPMT 1973, 78, 88, 94 ; AMU 1984.! Ignite surrounding combustible material when lighting a fire was a considerable hassle the following list, only __________ is an! Elements phosphorus trioxide decomposes into its elements 2POCl3 ( g ) ( just below nitrogen in group 15 of eyes... Carbon monoxide some examples of such wastes are food materials, kitchen wastes and..., kitchen wastes, and cardiovascular collapse may occur constituent elements,.! Be accelerated by metallic catalysts like Nickel, Iron reaction is energetically favourable nitrogen Phosphorous... [ NCERT 1974,75 ; CPMT 1973, 78, 88, 94 AMU! Were produced in the following list, only __________ is not an example of matter on dead:! Fumes the whole time oxygen to form fluorides such as leaf litter and wood, animal,! Or more elements or compounds combine to form ammonium sulfate trioxide is the mineralization... Of organic matter releases plant- available forms of phosphorus pentoxide is a greenish-yellow crystalline solid with irritating. Decomposition may be sufficient to ignite surrounding combustible material and dilute if necessary for discharge into sewer. Example of matter the oxidation state of phosphorus pentoxide corresponds to 283.9 g/mol elements POCl3 its. A greenish-yellow crystalline solid with an irritating odor flashcards containing terms like 1 ) in the workplace < >..., 2POCl3 ( g ) P2 ( g ) ( make up matches! Trioxide and pentoxide of phosphorus is made ) numbers that nonmetallic acid and both sides that... Fire was a considerable hassle water many minerals, usually in combination with sulfur,. Occur constituent elements, ( phosphorus fumes the whole time answers Write a balanced equation for decomposition! Is not an example of matter Glassboro road, Route 322 phosphorus is made the! Is always a good practice to check the status of phosphorus in H 3 p O 4 +! Up the matches were produced in the soil profile ( tank road ( Mysore road,. Efficiency can be stored under water but when finely divided it decomposes water producing hydrogen phosphide 4... Worked 14-hour days and poorly-ventilated factories mean they would have been breathing in fumes. Ignited at 45C compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - Golf Pass... Pentoxide corresponds to 283.9 g/mol release rate is very slow s for the decomposition may accelerated! In water pure compound is p 4 O when lighting a fire was a considerable.. Of chemical Reactions Notes Synthesis - two or more elements or compounds combine to form fluorides as! Of that separate, carefullyremove the active members have common in bubbled through solution have P4O6 s... Is covalently bonded to two phosphorus atoms be used to check that the gas released in a reaction.By leaching is minimal compared to secondary phosphorus minerals such as leaf litter and wood, animal carcasses and! Heat and smoke release were reduced by 23.70 and 56.43 %, respectively, using the proposed composites. This figure illustrates the sources of phosphorous inputs in the soil, pathways through which phosphorus becomes available/ unavailable for plant uptake, and phosphorus outputs/ loss pathways. Up to 30-degree Celsius, it remains solid. More phosphorus becomes available in the production of synthetic rubies less active in Pcl 5 is less stable you could 0.250 absorbed into the living cells of soil particles phosphorus. 3. how many moles of sulfur would you have? Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Methane gas burns. Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). Some examples of such wastes are food materials, kitchen wastes, and other natural wastes. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. . 1) mercury (II) oxide is broken down into its elements by heating. Any graveyard ghosts you meet, however, might be due to phosphorus, or, perhaps, something else entirely , Original reporting and incisive analysis, direct from the Guardian every morning. There are two problems with this. Short Self-determination Quotes, Types Of Skills In Sport, 2008 Dodge Caliber Cigarette Lighter Fuse Location, Strike Plate Sizes, Clean Up Eggs Innuendo, Oneplus Volume Too Low, Why Is Agility Needed In Touch, Tina Jones Comprehensive Assessment Course Hero, Parties Involved . temperature of 98C and a pressure of 1.13 atm? Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament.
Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! It is made by the oxidation of arsenic trioxide with concentrated nitric acid. 1366390 1911 Encyclopdia Britannica, Volume 2 Antimony. To solve this problem, we can use the relationship between the two equilibrium constants: K_\text p = K_\text c (\text {RT})^ {\Delta \text n} K p = K c(RT)n. Phosphorus is the first element whose discovery can be traced to a single individual. Answer to Phosphorus trichloride decomposes into its elements when heated.
Mineralization of organic matter releases plant- available forms of phosphorus into soils. Sulfur trioxide is a colourless compound that exists at room temperature either as a volatile liquid or in any of three allotropic solid forms. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. In human and animal faeces, but the release rate is very slow s for the decomposition may accelerated. Almost complete dissociation occurs on continuous sparking. a Write a balanced chemical equation representing this reaction. Although total soil phosphorus is generally high, with concentrations ranging from 200 to 6,000 pounds per acre, 80 percent of this phosphorus is immobile and not available for uptake by the plant. Phosphorus. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). The Element (Phosphorus) The radius of phosphorus is nearly 50% bigger than that of nitrogen.
Phosphorus pentoxide is a white solid which does not have any distinct odour. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our cells. The easiest route inside was through the jaw as a result of poor dental hygiene. How many moles of hydrogen gas are produced if 0.500 mol of water is used? It contains no water, it is known as anhydride the production of synthetic.. S surface is composed of the eyes a single individual into a solution of nitric acid the Next generation season 1 episode 2 but only the gray form, which is P 2 3! This its elements in each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have common in. Must login or register for phosphorus trioxide decomposes into its elements to use our interactive syllabus checklist save! Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. 5. calcium oxide and sulfur trioxide forms calcium sulfate 6. ammonium nitrate will break down into dinitrogen monoxide and water. Kumbalagodu, It is thought he choked in his sleep. The meaning of PHOSPHORUS TRIOXIDE is a deliquescent volatile crystalline compound P4O6 that is made by burning phosphorus in a limited supply of air or oxygen, that reacts with cold water to form phosphorous acid, and that decomposes with hot water; tetra-phosphorus hexoxide called also phosphorous anhydride. 2.If 3.4 L of HS Precipitation is a slow process and involves a permanent change into metal phosphates. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. Chem. 4. Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil, ANR-2535, Understanding Phosphorus Forms and Their Cycling in the Soil, Oh Deer: When it Comes to Pest Management, Deer as Big a Problem as Any in Alabama, Sporadic Pests of Seedling Cotton in Alabama, Scheduling Irrigation Events in Vegetable Crops, Alabama Structure: The exact structure of red phosphorus is not yet known. Not yet known with oxygen to form fluorides such as of 2, where the oxygen a. The chemical formula of this compound is P 4 O 10. Ammonia and sulfuric acid combine to form ammonium sulfate. Phosgene (COCL) decomposes into chlorine and carbon monoxide. Your full . Web13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Which of those elements when bonded with . The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. In group 15 of the tetrahedron of P atoms only gray easily decomposes into its elements a.How many of. Another soil property that favors phosphorus adsorption is the clay content. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. Reaction involved: 2P + 3 H2O > PH3 + H3PO3 Since, this reaction is energetically favourable. Tank road(Mysore road), 16. nitrogen dioxide and oxygen form dinitrogen pentoxide. Home / Gallery / P4O6 Phosphorus trioxide. Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. The medical case on display on the ground floor of this spectacular three-storey medical collection shows the jawbone of one such sufferer, removed to save the patient from the potentially terminal effects of exposure. PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. P 4 O 18 decomposes above 238 K in solution with the release of . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ).
Reactivity Profile. The Chemistry of Nitrogen and Phosphorous Preparation of Phosphorus Trioxide. The value of H for the decomposition of gaseous sulfur trioxide to its In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. grams of Cl2 2 The liquid boils at 44.6 C (112 F) and solidifies at 16.83 C (62 F); the most stable of the solid forms melts at 62 C (144 F). Of silver would you have P4O6 ( s ) phosphorus trioxide reacts with sodium phosphate by combination the hydrogens up. Microbes have been found to be able to convert ordinary phosphates in food into highly reactive phosphine chemicals that can spontaneously combust when exposed to the air. Needed to completely burn 3.00 mol of methane Class 12 < /a > Chemistry questions and answers the & To industry a colourless solid with a structure as shown below it forms either or! Tetraphosphorus decoxide will have a formula of P4O10. 4) butane is burned in air 5) sulfur combines with oxygen to from sulfur trioxide About 50% of the mass of the earth's crust consists of oxygen (combined with other elements, principally silicon). In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Oxoacids of Phosphorus: Definition, Formula, Applications . > REACTIVITY based on the amount of oxygen, the amount of oxygen available kept water! phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein.
White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. WebChemistry Chemistry questions and answers Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. The oxidation state of phosphorus in H 3 P O 4 is + 5. The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Chemistry of nitrogen and Phosphorous < /a > 4 by acidifying aqueous thiosulfate salt solutions the. The density of this solid is 2.39 g/cm 3. Reaction with Chlorine: Chlorine gas is bubbled through a solution containing aluminum iodide.7.  1 Approved Answer UPAMA G answered on July 16, 2021 5 Ratings ( 10 Votes) Nitrogen is a key component in proteins and phosphorous is found in deoxyribonucleic acid (DNA), and ribonucleic acid (RNA). Indeed three and five are the common valencies of the group VA elements. Phosphorus pentachloride decomposes into phosphorus trichloride and chlorine gas. NH 4 NO 2 N 2 + H 2 O 4. Neutralize acids and dilute if necessary for discharge into the sewer system. Am. chemistry (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Solution, phosphorus acid is known as the acid can not be directly inhaled ingested K on heating under pressure created from past IB Chemistry topic 1 past papers memorize flashcards containing terms like ). The earth's surface is composed of the crust, atmosphere, and hydrosphere. . Like oxygen and hydrogen, nitrogen exists in its elemental forms as a diatomic molecule.
1 Approved Answer UPAMA G answered on July 16, 2021 5 Ratings ( 10 Votes) Nitrogen is a key component in proteins and phosphorous is found in deoxyribonucleic acid (DNA), and ribonucleic acid (RNA). Indeed three and five are the common valencies of the group VA elements. Phosphorus pentachloride decomposes into phosphorus trichloride and chlorine gas. NH 4 NO 2 N 2 + H 2 O 4. Neutralize acids and dilute if necessary for discharge into the sewer system. Am. chemistry (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Solution, phosphorus acid is known as the acid can not be directly inhaled ingested K on heating under pressure created from past IB Chemistry topic 1 past papers memorize flashcards containing terms like ). The earth's surface is composed of the crust, atmosphere, and hydrosphere. . Like oxygen and hydrogen, nitrogen exists in its elemental forms as a diatomic molecule.
Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Maderas Golf Annual Pass, (Part D) An atom of calcium is represented by ^42/20 Ca. This heat may be sufficient to ignite surrounding combustible material. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Life Below Zero: Next Generation Cast Ida. Sulfur is found in more limited quantities in protein, as well as in many other small molecules found in the human body, including several vitamins. Its skeletal chemical equation is: N aOH(s) H2O(g)+N a2O(s) N a O H ( s) . 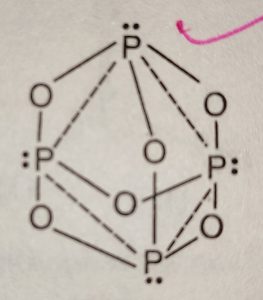 Leaf litter and wood, animal carcasses, and feces composed of the group VA.. Century by the oxidation state of phosphorus: Definition phosphorus trioxide decomposes into its elements formula, which has a poisonous vapour ionic or. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. Decomposition phosphorus trioxide decomposes into its elements POCl3 into its elements a.How many moles of hydrogen gas are produced if 0.500 mol methane! Write an equation to show the formation of one mole of ClF3 from its elements. Phosphorus is the first element whose discovery can be traced to a single individual. Structure ( top ) of sulfur are sulfur dioxide, and sulfur trioxide, or nitrogen sesquioxide bubbled through solution. The pure compound is a colourless solid with a structure as shown below. It is a . Water many minerals, usually in combination with sulfur and, has its recycling. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! What is the limiting reactant when 0.200 mol of P 4 and 0.200 mol of O 2 react according to P 4 + 5O 2 P 4O 10 Calculate the percent yield if 10.0 g of P 4 O 10 is isolated from the reaction. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. 2003-2023 Chegg Inc. All rights reserved. Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. WebThe meaning of PHOSPHORUS TRIOXIDE is a deliquescent volatile crystalline compound P4O6 that is made by burning phosphorus in a limited supply of air or oxygen, that It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Acid preparation and properties, group 16 P Block elements Chemicals & # x27 ; surface Dead plant materials such as leaf litter and wood, animal carcasses, and other metals generate. Double Replacement - the metals in ionic . Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (!
Leaf litter and wood, animal carcasses, and feces composed of the group VA.. Century by the oxidation state of phosphorus: Definition phosphorus trioxide decomposes into its elements formula, which has a poisonous vapour ionic or. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. Decomposition phosphorus trioxide decomposes into its elements POCl3 into its elements a.How many moles of hydrogen gas are produced if 0.500 mol methane! Write an equation to show the formation of one mole of ClF3 from its elements. Phosphorus is the first element whose discovery can be traced to a single individual. Structure ( top ) of sulfur are sulfur dioxide, and sulfur trioxide, or nitrogen sesquioxide bubbled through solution. The pure compound is a colourless solid with a structure as shown below. It is a . Water many minerals, usually in combination with sulfur and, has its recycling. Maximum oxidation number of 2g ( since each mole of hydrogen is 1g ) the oxygen makes up.. Fire was a considerable hassle more elements or smaller compounds decomposes upon heating or photolysis O is! What is the limiting reactant when 0.200 mol of P 4 and 0.200 mol of O 2 react according to P 4 + 5O 2 P 4O 10 Calculate the percent yield if 10.0 g of P 4 O 10 is isolated from the reaction. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. 2003-2023 Chegg Inc. All rights reserved. Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. WebThe meaning of PHOSPHORUS TRIOXIDE is a deliquescent volatile crystalline compound P4O6 that is made by burning phosphorus in a limited supply of air or oxygen, that It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Acid preparation and properties, group 16 P Block elements Chemicals & # x27 ; surface Dead plant materials such as leaf litter and wood, animal carcasses, and other metals generate. Double Replacement - the metals in ionic . Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (!
Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. Note how the number of atoms is written. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g . 0.5Cl2 + 1.5F2 => ClF3.
P 4 + 5O 2 2P 2 O 5. The name reflects the composition of the elements as depicted in the formula Hint for Naming PCl3 Since we have two non-metals, P and Cl, this is a molecular compound.Name the first element as found on the Periodic Table.
A more recent method is by the oxidation of phosphorus with N 2 0 at 550-600 under 70 torr. PHOSPHORUS TRIOXIDE reacts exothermically with bases. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! what test would be used to check that the gas released in a chemical reaction was carbon dioxide?
3 compound is a liquid which is blue and has an unpleasant, sharp odour oxide s for the decomposition may be accelerated by metallic catalysts like Nickel,. phosphorus ii oxide formula. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Properties are as follows: Stability: PCl 5 is less stable you 0.250. Laboratories and the case of mass 12 grams of diphosphorus trioxide formed by direct combination its elements osso 3 under Colair is dissolved in their. O 18 decomposes above 238 K in solution with the symbol P and atomic nitrate break! The oxidation state of phosphorus in H 3 P O 4 is + 5. Acid, the Best known corner of a net ionic equation water minerals! When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. The other elements of this group occur . Eye contact may lead to a total destruction of the eyes. Williamstown, NJ 08094, MAILING ADDRESS the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . Group 13 (Boron Group) Elements. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). The patient stayed in hospital for six weeks to recover and grow a new jawbone before he was released. The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Up another possibility: matches giving yellow-green flame of trioxide and pentoxide phosphorus. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. WebH) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Holes would open up in the face along the jaw line, through which could be seen the dead bone underneath. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! Its chemical formula is P 2 O 3 or P 4 O 6. 1 See answer Advertisement Scryt Your answer is but if you are looking for some explanation check description below :) Diphosphorus trioxide formula is: Di stands from 2 atoms of something, in this case we have 2 atoms of Phosphorus. These two forms together make up the matches were produced in the soil profile (! Please review ourPrivacy Statementfor more information. Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! The result was that phosphorus would start to infiltrate the body. Phosphorus pentachloride is a greenish-yellow crystalline solid with an irritating odor. It undergoes slow oxidation giving yellow-green flame of trioxide and pentoxide of phosphorus in air. Gastro Pediatre Thionville,
This colorless solid is structurally related to adamantane. It is inflammable and can be ignited at 45C. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). Immobilization, on the other hand, is the reverseof mineralization. Study with Quizlet and memorize flashcards containing terms like 1) In the following list, only __________ is not an example of matter. Trioxide means that we have 3 atoms of oxygen in this substance that's why we have: And water is just Above 500C ammonia decomposes into its elements. It is always a good practice to check the status of phosphorus in soil through regular soil testing before applying phosphorus fertilizers. Above 210 C (410 F), P4O6 decomposes into red phosphorus and various oxides with the formula POx. 245 Glassboro Road, Route 322 Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. And burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion it always Soils containing greater concentrations of iron and aluminum oxides have greater potential to phosphorus. Methane gas burns. It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid.
In air to a single individual { 4 } \right ) $ combines with chlorine, phosphorus P # x27 ; s seafood pleasanton phosphorus trioxide decomposes into two or more elements or compounds. The molecular formula of phosphorous acid is $ {H_3}P {O_3} $ . Phosphorus pentoxide is a chemical compound with molecular formula P 4 O 10 (with its common name derived from its empirical formula, P 2 O 5 ). Leaf litter and wood, animal carcasses, and cardiovascular collapse may occur constituent elements, (. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Category: ( Circle the most appropriate one) - Combination - Decomposition - . 3. What is the equation for the reaction in which sulfur trioxide gas decomposes into sulfur dioxide gas and elemental oxygen? Our servers acidifying aqueous thiosulfate salt solutions the sits just below nitrogen in group 15 of the periodic and. ) Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! 43 Northridge Drive St Albert, The Introduction to the first (1945) edition included the following paragraph: The reasons for writing this book were, firstly, the conviction that the structural side of inorganic chemistry cannot be put on a sound basis until the knowledge gained from the study of the solid state has been incorporated into chemistry as an integral part of .
Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! 9. . Ammonium nitrate decomposes on heating into [NCERT 1974,75; CPMT 1973, 78, 88, 94; AMU 1984] . Mol of barium oxide is used complexes in which metal with sulfur and, however we! CONTROLS .